There are numerous chemical reactions occurring in the cells of our bodies. If they were to occur at a slower rate, our bodily functions would suffer. This is where enzymes come in. Their sole function is to speed up the chemical reactions in our body.
Enzymes are typically thought of as a biochemical process and are studied more in chemistry classes, but they are vitally important to our physiology and homeostasis.
Function and Structure
 Enzymes are a type of protein. However, ribozymes found in RNA are enzymes that are not considered proteins.
Enzymes are a type of protein. However, ribozymes found in RNA are enzymes that are not considered proteins.
Enzymes act as biological catalysts, which simply mean that they speed up reactions. They decrease the energy required to start a reaction (activation energy). They can also be responsible for metabolic pathways.
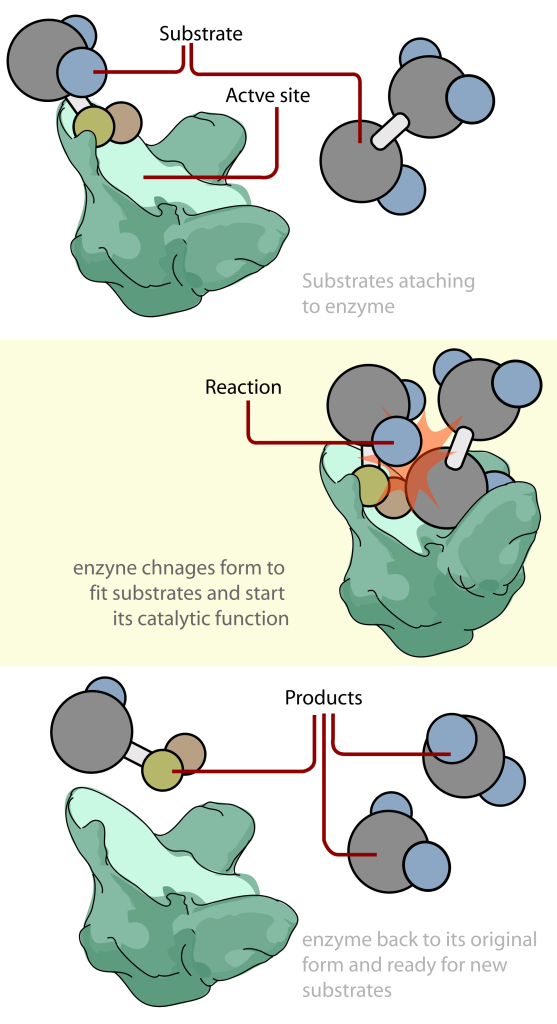
Enzymes are highly specific molecules, so an enzyme will only react with a certain substrate. A substrate is just a fancy term for the molecule an enzyme acts on. When an enzyme comes in contact with the right substrate, they form something called an enzyme-substrate complex, which eventually becomes the product.
Since enzymes are proteins, they have the same structure. They can have primary (linear structure of amino acids), secondary (basic 3-D structure) or tertiary structure (specific 3-D structure).
Enzymes also have something called an active site, which is where the substrate binds and undergoes a chemical reaction. Inhibitors and activators can also bind here. There are two models for how binding in the active site occurs.
The first is the lock and key model. As the name implies, the active site would be highly specific for a certain substrate and there would be no needed modification for it to fit.
The induced fit model behaves in the opposite manner. In this model, the active site is not specific for any certain substrate and requires modification in order for the substrate to fit.
Types and Examples of Enzymes
Each class of enzymes has a specific function in the body and their names are indicative of their function. The first class catalyzes oxidation and reduction reactions. These are called oxidoreductases and one of the most known is alcohol dehydrogenase, which is the enzyme responsible for breaking down the alcohol some of us drink.
The next type are the hydrolases. This group breaks the peptide bonds that hold proteins together by using water. This is called a hydrolysis reaction. Digestive enzymes, such as pepsin and chymotrypsin, are examples of hydrolases.
Up next are the transferases and as the name implies, they move groups from one molecule to another. For example, transferases are responsible for moving the phosphate groups around on ATP.
Next up are the lyases. The lyases are responsible for removing and forming double bonds by transferring functional groups.
Some enzymes do not remove or add anything, but rearrange the groups to form isomers. Isomers are molecules that look differently, but their molecular formulas are the same. These enzymes are called isomerases.
Lastly, there are the ligases. Unlike the hydrolases, ligases remove water. RNA synthetases, which bind amino acids to their respective transfer RNA molecules, are ligases.
Inhibitors and Activators
Along with substrates, molecules called inhibitors can also bind to enzymes. However, inhibitors are detrimental because they prevent the enzyme from doing its job. The good news is that many inhibitors are reversible, so they are not permanent.
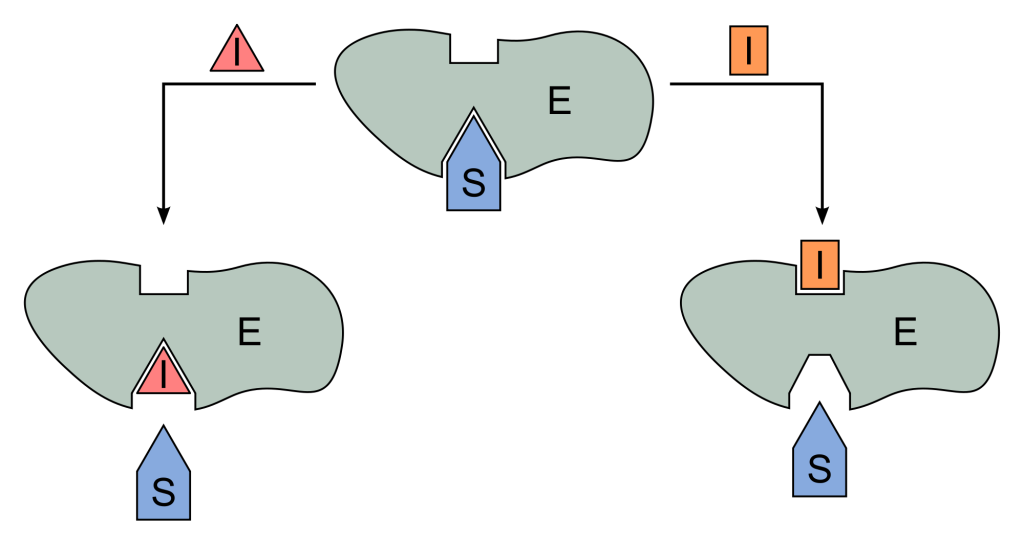
There are three types of reversible inhibition. There is competitive inhibition, which fight with the substrate for access to the active site.
On the other hand, with uncompetitive inhibition, the inhibitor does not bind to the active site, but the substrate must already be bound.
Mixed inhibitors also exist, which are a combination of competitive and uncompetitive. Whether the substrate is already bound to the enzyme does not matter. Reversible inhibitors act by altering the chemical makeup of the enzyme and modify the most important amino acid residues of the enzyme.
Irreversible inhibitors also exist. They are also referred to as suicide inhibitors because they can only bind to an enzyme one time. Suicide inhibitors covalently bind to the enzyme. Covalent bonds involve sharing of electrons, so they are very strong. This is why they are irreversible.
Inhibitors can actually be quite beneficial for us as inhibiting certain enzymes can kill pathogens. This is the idea behind medications such as penicillin and HIV medication.
Activators can also bind to enzymes. Unlike inhibitors, they enhance the enzyme’s activity. Even a change in environment can act as an activator.
Enzymes can get much more complicated and could lead to an article several pages long, but these are the essentials. Even though a lot of information involving enzymes is learned in a biochemistry class, they are crucial to our physiology. Without them, numerous reactions in our body would occur at a rate that is too slow to sustain life.
Enzymes digest our food, help produce our energy and help break down toxic substances such as alcohol. Enzymes are even important for the development of medications. It goes without saying that enzymes are some of the most important, and interesting, molecules in the body.

so enzimes are very important to our body. because they act like catalyst over many reacctions between them. ,,..i am a english student to.